Learning Outcomes
By the end of this lesson, students should be able to:
i. Explain the fundamental principles of proton NMR (nuclear magnetic resonance) spectroscopy, including the interaction of hydrogen nuclei with magnetic fields and the concept of resonance.
ii. Describe the factors influencing chemical shift, the key parameter for identifying protons in NMR spectra, such as electron density and electronegativity of neighboring atoms.
iii. Interpret proton NMR spectra to determine the chemical shift, multiplicity (number of peaks), and integration (relative abundance) of protons in different environments within a molecule.
iv. Apply their knowledge of proton NMR spectroscopy to identify the structure of simple organic compounds based on their NMR spectra.
v. Appreciate the power of proton NMR spectroscopy as a versatile tool for elucidating molecular structures in organic chemistry.
Introduction
Proton NMR (nuclear magnetic resonance) spectroscopy, a cornerstone of organic chemistry, provides detailed information about the structure and connectivity of organic molecules by analyzing the magnetic resonance of hydrogen (proton) nuclei. This lesson delves into the principles of proton NMR spectroscopy, guiding students through the concepts of resonance, chemical shift, multiplicity, and integration, enabling them to interpret NMR spectra and extract structural information.
i. The Magnetic Symphony: Proton Resonance
When hydrogen nuclei, with their inherent magnetic moments, are placed in a strong magnetic field, they undergo a phenomenon called resonance. This resonance occurs when the energy of an applied radiofrequency pulse matches the energy difference between the aligned and non-aligned states of the proton's magnetic moment.
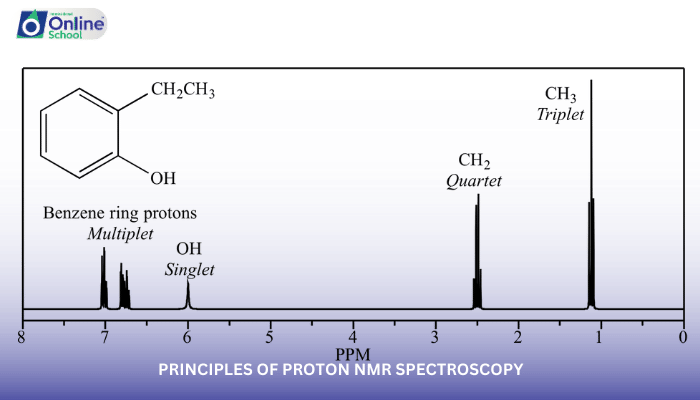
Chemical Shift: The Fingerprint of Proton Environments
The position of an NMR absorption peak, known as the chemical shift, is directly related to the magnetic environment of the proton. Electron density around the proton influences its chemical shift, with protons in more electron-dense environments experiencing higher chemical shifts.
Multiplicity: Deciphering the Neighborhood
The number of peaks for a particular proton in an NMR spectrum, known as the multiplicity, provides information about the number of equivalent protons in its neighborhood. Splitting patterns, such as triplets, quartets, and quintets, arise from interactions with neighboring protons.
Integration: Unveiling Relative Abundance
The area under an NMR absorption peak, known as the integration, reflects the relative abundance of protons in a particular environment. This information is crucial for determining the stoichiometry of protons within a molecule.
ii. Interpreting NMR Spectra: A Structural Puzzle
Interpreting proton NMR spectra involves analyzing chemical shift, multiplicity, and integration to determine the structure of a molecule:
Identify Chemical Shifts: Assign chemical shifts to different types of protons based on their electron environment.
Determine Multiplicity: Analyze splitting patterns to deduce the number of equivalent protons and neighboring protons.
Relate Integration to Structure: Determine the relative abundance of protons using integration values.
Piece Together the Puzzle: Combine information from chemical shift, multiplicity, and integration to construct the molecular structure.
Proton NMR spectroscopy, with its ability to reveal the magnetic resonance of hydrogen nuclei and provide detailed insights into chemical shift, multiplicity, and integration, has become an indispensable tool in organic chemistry. By interpreting proton NMR spectra, chemists can decipher the structure and connectivity of organic compounds, unlocking the secrets of their molecular architecture.